Research Highlights
Article |
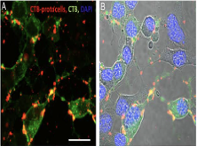
In the present study, the endocytic mechanism and intracellular fate of CTB-
protocells in motoneurons were examined to provide information for the development of therapeutic application and cargo delivery.
Porras, Maria A. Gonzalez; Durfee, Paul; Giambini, Sebastian; et al.
Article |
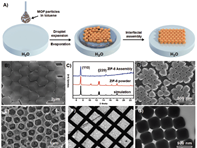
DPGG (1,2-dipalmitoyl- sn-glycero-3-galloyl) with metal nodes/sites surrounding MOF surface. X-ray diffraction and Argon sorption analysis prove that the modified MOF particles retain their structural integrity and porosity after surface modification.
Wei Zhu, Guolei Xiang, Jin Shang, Jimin Guo, Benyamin Motevalli, Paul Durfee,
Jacob Ongudi Agola, Eric N. Coker, and C. Jeffrey BrinkerArticle |
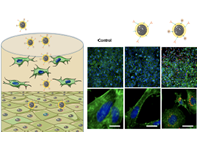
Multifunctional Protocells for Enhanced Penetration in 3D Extracellular Tumoral Matrices
The use of proteolytic enzymes prior to nanoparticle administration or directly attached to the nanocarrier surface has been proposed to enhance their penetration, but the low in vivo stability of these macromolecules compromises their efficacy and strongly limits their application.
María Rocío Villegas,Alejandro Baeza,*Achraf Noureddine,§ Paul N. Durfee,§,⊥ Kimberly S. Butler,§, Jacob Ongudi Agola,§,⊥ C. Jeffrey Brinker,*, and María Vallet-Regí
María Rocío Villegas,Alejandro Baeza,*Achraf Noureddine,§ Paul N. Durfee,§,⊥ Kimberly S. Butler,§, Jacob Ongudi Agola,§,⊥ C. Jeffrey Brinker,*, and María Vallet-Regí
Article |
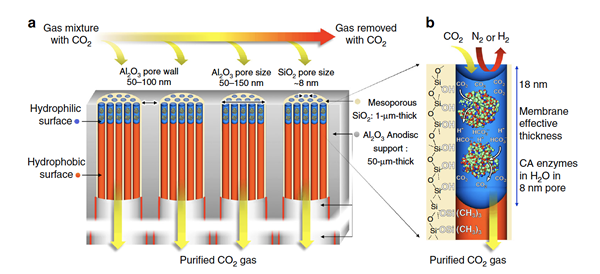 Ultra-thin enzymatic liquid membrane for CO2 separation and capture
Ultra-thin enzymatic liquid membrane for CO2 separation and capture
Here we describe an enzymatically active, ultra-thin, biomimetic membrane enabling CO2 capture and separation under ambient pressure and temperature conditions.
Yaqin Fu1,2, Ying-Bing Jiang1,2,3, Darren Dunphy1,2, Haifeng Xiong 1,2, Eric Coker 4, Stan Chou4 Hongxia Zhang5, Juan M. Vanegas4,6, Jonas G. Croissant1,2, Joseph L. Cecchi1, Susan B. Rempe 4 & C. Jeffrey Brinker1,2,4
Yaqin Fu1,2, Ying-Bing Jiang1,2,3, Darren Dunphy1,2, Haifeng Xiong 1,2, Eric Coker 4, Stan Chou4 Hongxia Zhang5, Juan M. Vanegas4,6, Jonas G. Croissant1,2, Joseph L. Cecchi1, Susan B. Rempe 4 & C. Jeffrey Brinker1,2,4